
The surprising results of Rutherford, which we will explore in more detail in the next section, indicated that the positively charged subatomic particles-the protons (p +)-are much more massive than electrons (by a factor of about 2,000) and are highly concentrated in the atom’s nucl e us, which occupies just a tiny portion of the atom’s entire volume. We designate electrons with the symbol e –, the superscript emphasizes their negative charge. Scientists established that the atom was not indivisible and due to the work of Thomson, Millikan, and others, the charge and mass of the negative, subatomic particles-the electrons-were known. | Key Concepts and Summary | Glossary | End of Section Exercises | The Structure of the Atom In the early part of the 20th century much of our current understanding of atoms and atomic structure had been established.
Krypton number of neutrons update#
Wapstra, "The 1995 Update to Atomic Mass Evaluation," Nuclear Physics, A595:409–480 (1995) K.J.R. The numerical difference between the actual measured mass of an isotope and A is called either the mass excess or the mass defect (symbol Δ see table). The total number of neutrons and protons (symbol A), or mass number, of the nucleus gives approximately the mass measured on the so-called atomic-mass-unit (amu) scale. Many important properties of an isotope depend on its mass. The three share the place in the periodic table assigned to atomic number 1 and hence are called isotopes (from the Greek isos, meaning “same,” and topos, signifying “place”) of hydrogen. Three nuclei with one proton are known that contain 0, 1, and 2 neutrons, respectively. In fact, it is precisely the variation in the number of neutrons in the nuclei of atoms that gives rise to isotopes. Not all the atoms of an element need have the same number of neutrons in their nuclei. The periodic table of the elements assigns one place to every atomic number, and each of these places is labeled with the common name of the element, as, for example, calcium, radon, or uranium. A bar of pure uranium, for instance, would consist entirely of atoms with atomic number 92. A large collection of atoms with the same atomic number constitutes a sample of an element. The great importance of the atomic number derives from the observation that all atoms with the same atomic number have nearly, if not precisely, identical chemical properties. This atomic number is ordinarily given the symbol Z. Every chemical element has one or more isotopes.Īn atom is first identified and labeled according to the number of protons in its nucleus. Isotope, one of two or more species of atoms of a chemical element with the same atomic number and position in the periodic table and nearly identical chemical behaviour but with different atomic masses and physical properties. SpaceNext50 Britannica presents SpaceNext50, From the race to the Moon to space stewardship, we explore a wide range of subjects that feed our curiosity about space!.Learn about the major environmental problems facing our planet and what can be done about them!

Saving Earth Britannica Presents Earth’s To-Do List for the 21st Century.Britannica Beyond We’ve created a new place where questions are at the center of learning.100 Women Britannica celebrates the centennial of the Nineteenth Amendment, highlighting suffragists and history-making politicians.

Krypton number of neutrons how to#

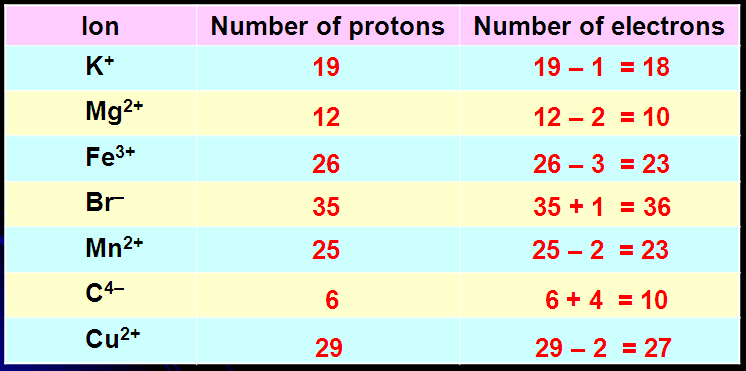
#WTFact Videos In #WTFact Britannica shares some of the most bizarre facts we can find.Demystified Videos In Demystified, Britannica has all the answers to your burning questions.Britannica Explains In these videos, Britannica explains a variety of topics and answers frequently asked questions.Britannica Classics Check out these retro videos from Encyclopedia Britannica’s archives.


 0 kommentar(er)
0 kommentar(er)
